1% of the population averages 6.25 hours of sleep a night.
They don’t choose to sleep less — they have a mutation(s) that allows them to naturally wake up at 5am feeling well rested.
A 10 year study lead by Ying-Hui Fu and Louis Ptáček uncovered the cellular level processes that lead to the condition. There are three known mutations.
DEC2
MyoD1 is a gene that encodes a protein that promotes orexin production. Orexin is a neuropeptide that regulates arousal, wakefulness, and appetite. DEC2 is a protein that binds to MyoD1 to inhibit transcription by RNA polymerase.
When DEC2 functions properly, orexin inhibition is in sync with the circadian rhythm. As it gets dark outside, your brain produces melatonin in the pineal gland, which basically inhibits certain parts of the brain when you fall asleep.
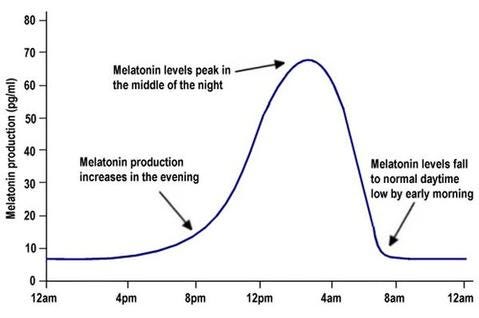
Flux of DEC2 production is very similar to that of melatonin’s. During the day, production is low. During the night, production is high.
But for the 1% of people that have the mutation, their DEC2 proteins don’t bind very strongly to MyoD1, meaning RNA polymerase can still synthesize the protein that increases orexin production. This effects orexin production closer to dawn, when DEC2 production starts to decrease.
ADRB1
The ADRB1 gene encodes the protein beta-1 adrenergic receptor. Beta-1 adrenergic receptors act as neuron inhibitors — when they receive agonist chemical signals, they inhibit major processes in the neuron. When ADAB1 is mutated, beta-1 adrenergic receptor degrades much faster. As they degrade, they lose their ability to inhibit function, meaning neurons function longer, and a person can stay awake longer.
NPSR1
They had discovered DEC2 and ADRB1 mutations in most patients with the condition, but couldn’t find it in some. So they took a family that all had the condition but didn’t have DEC2 or ADRB1 mutations and looked at their dorsal pons brain tissue. They found a mutation in NPSR1.
NPSR1 encodes a G protein-coupled receptor that binds to neuropeptide S (NPS). NPS is mainly produced in the amygdala and spreads within the Thalamus region. It induces wakefulness and arousal.
Those with the mutation have more sensitive receptors, meaning they feel more awake with low levels of NPS. They inflicted mice with the mutation to validate that it had something to do with sleep. They found that those mice slept 59min less on average (52min less deep sleep and 7min less REM sleep) while still waking up rested.
Effect
Sleep deprivation effects cognitive performance and health poorly.
1) It reduces cognitive abilities. There are lots of studies showing decrease in performance the longer the cumulative deprivation. Here’s a graph taken from this paper. (Black squares = 0hrs, Circles = 4hrs, White squares = 6hrs, Diamonds = 8hrs).
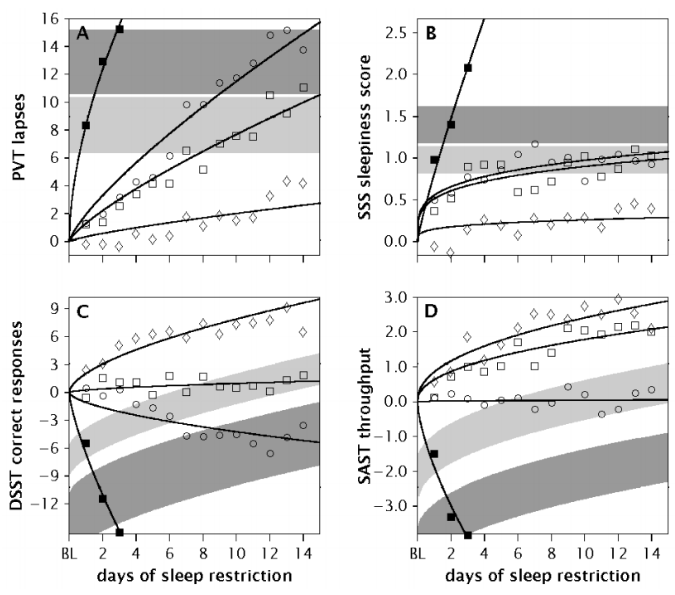
- PVT lapses → lapses in psychomotor vigilance task (basically the amount of times you stop focusing on something that you’re supposed to, like “follow this dot as it moves around the screen”).
- DSST → digit symbol substitution task (basically substituting symbols in a digital graph)
2) Increases mortality rate by 13% (if you want to look more into this, I made a paper here).
You would expect people with these mutations to have terrible cognitive abilities and be riddled with health conditions, but they’re not.
There isn’t as much research in this space, but Ying-Hui Fu and Louis Ptáček claim these mutations don’t cause any significant health defects. Some studies even show they increase life span (although sample sizes are small).
Implication
If these mutations are only beneficial, why wouldn’t we all have them? The leading theory is that it they only came up a few thousand years ago. Because of technological progression, it hasn’t had large effects on evolution (we aren’t struggling for food, we aren’t struggling to survive, reduction of sleep doesn’t effect us as much as it would have 200K years ago).
Inducing a mutation is very invasive. It’s also pretty much impossible to do in all neurons in the brain of a full grown adult. Alternatively, one of the most popular non invasive ways to influence neuron activity is transcranial stimulation.
If we could reduce the inhibitory effects of DEC2, reduce the level of inhibitive capabilities of beta-1 adrenergic receptors (involved in ADRB1), or increase the sensitivity of NPSR1 G protein encoded receptors to NPS, we could reduce sleep time without harming cognitive processes or long term health.